17th October 2023
Extracting limonene from citrus peel
The chemistry of extracting substances from plant materials is important because many such compounds are physically active, either medically or aesthetically (e.g. perfumes). Recently, A2 members of our Chemistry Society extracted limonene from orange peel using a refluxing technique that was first introduced to them last year as part of their core practical skills.
Limonene is an alkene (containing C=C bonds) that is naturally found in orange peel and gives it its zesty aroma. Some background chemistry of limonene is shown below.
Limonene
The basic building block for many natural products is isoprene (C5H8) (Fig a). In fact, when it fully polymerises, it forms latex.
The molecular formula for limonene is (C10H16), consisting of 2 isoprene units linked together (Fig b). Making models of limonene with Molymods shows that the arrangement around the red bond (the bond on the right hand side of Figs b and c) can either come out of the plane of the paper (Fig c) or go behind the plane of the
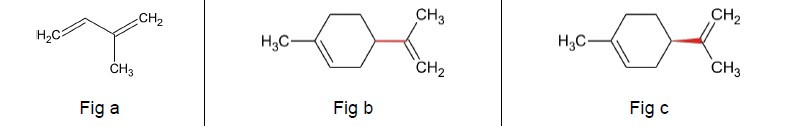
paper (Fig b). These chemicals are called stereoisomers. The D-form is the one predominant in oranges whereas the L-form is found more as an oil in pine trees and has a different smell. Nature is a superb chemist!
Part 1:
Refluxing the peel to extract the limonene
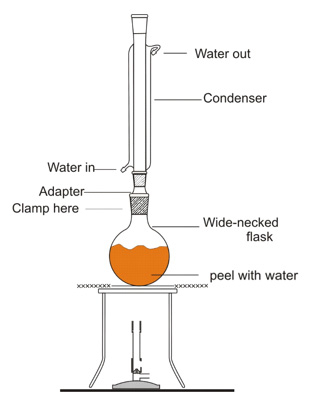

Above: Oliver and Peter set up their refluxing apparatus.

Above: Summer and Olivia set up their refluxing apparatus.

Above: George keeps an eye on his refluxing apparatus once it’s being heated. You can clearly see the colour from the orange peel added to the water in the pear-shaped flask.

Above: George and Hector discuss whether it’s refluxed for long enough before re-arranging the apparatus for distillation.
Part 2 Distilling the limonene
In this part, the students separated the limonene from the water in which the peel was placed, based on their different boiling points. In the picture below, Tom checks for the appearance of distillate in the collecting vessel (to the right of the apparatus). Scroll down for a diagram of the apparatus.

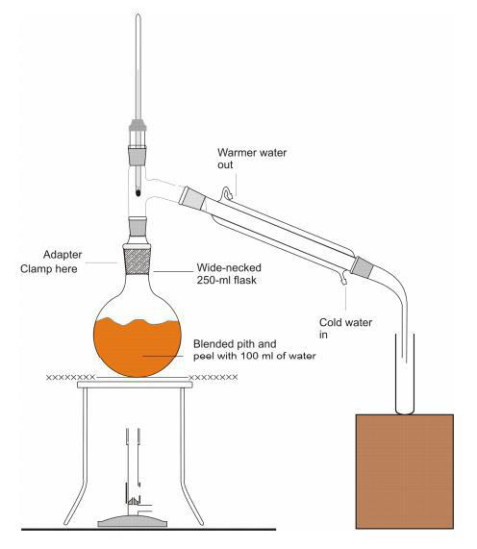

Above: Olivia and Summer wait for their final product (below) to start collecting in the measuring cylinder during distillation.

Above: The collected product ready to separate out. In theory, most of this will be limonene but in practice, it’s actually mostly water, so further purification is needed.
Part 3: Separating the limonene from the mixture using a separating funnel
Despite the distillation, some water will have made it all the way through to the collecting vessel. This needs to be further purified in this part, the limonene molecules do not mix with water molecules due to incompatible intermolecular forces between the two molecules. We use a separating funnel (below) to do this.

Above: Limonene layer (lower density so floats on top) Aqueous layer (higher density so sinks to the bottom)
Part 4 Thin-layer chromatography
Here, we use the fact that limonene and any other organic compounds present will be separated due to varying degrees of intermolecular forces for the TLC “plate” compared with the solvent.
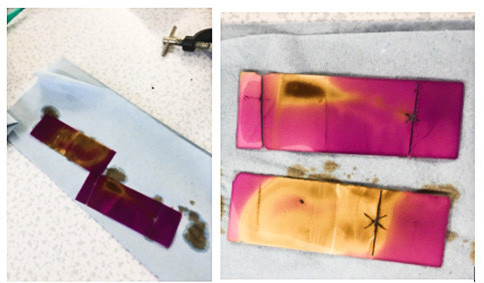
Above (L-R) : The finished TLC plate is dipped in potassium manganate (VII) solution to colour the components. As an oxidising agent it reacts with them and changes colour itself. This is then dried using a hairdryer before the results reveal themselves (see below)

Above left: This is a chromatogram (finished TLC plate) showing an actual result.
Above right: This is a chromatogram of pure limonene for comparison. You can see the position of the limonene spot in both.

Above: This specialised jar is used to keep the TLC plate upright, allowing the solvent to rise up along its length, carrying components as it goes. The lid prevents the solvent from evaporating.
