04th January 2024
Aspirin synthesis and analysis – November 2023
Throughout the month of November our Chemistry Society made, purified and tested the chemical composition of aspirin. Aspirin is the common “trade name” for 2-ethyloxybenzoic acid (below).
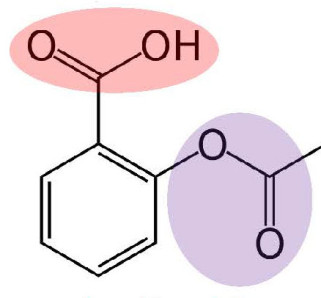
This compound contains the carboxylic acid and ester functional groups, both of which are covered in the A level chemistry syllabus. A video taking you through the chemistry of the process of making this substance can be found here.
A flavour of the three week Chemistry Society project can be found in the photos below!
Week one: Synthesis using refluxing of methyl-2-hydroxybenzoate.

The reaction mixture is heated for 30 minutes.
Week two – recrystallisation and purification.

Hector and George set up their purification using reduced pressure vacuum filtration.

Eva washes her final product after filtration.

Eva’s yield of solid product is impressive!

Peter sets up his ice bath to cool his reaction mixture down after recrystallisation. The idea is that pure aspirin will crystallise out.
Week 3 – testing for purity.

Iron(III) sulfate can be used to test for the presence of a phenol. The starting material was sodium 2-hydroxybenzoate (see video) – the purple colour shows that in the impure aspirin product, some of this is left behind.

You can see the quality of Katie’s final product – the needle shaped crystals are the shape adopted by pure solid aspirin.
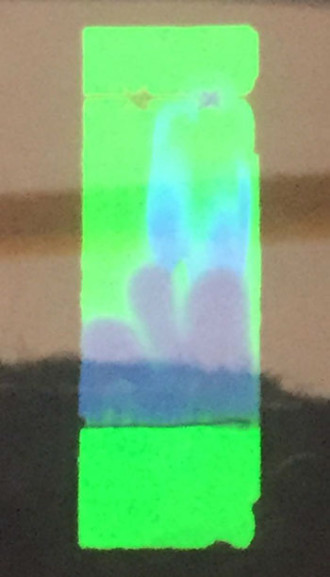
Under UV light, you can see clearly George’s TLC chromatogram..

..which he then marked and measured..

..recorded and finally used to calculate the Rf value (next page) for his aspirin.

